|
In 2006-07, foreign direct
investment (FDI) into India tripled to $16 billion (Rs 67,200
crore) and nearly half (48.5 per cent) of it came from us companies.
This, argues Pamela Parizek, Director, KPMG, Washington (above),
is justification enough for Indian companies listed on us exchanges
or doing business there, to comply with the provisions of the
Foreign Corrupt Practice Act (FCPA), a US law. "FCPA seeks
to place us companies operating in foreign countries and foreign
companies listed on us exchanges or operating there on a level
playing field with respect to compliance issues," says Parizek,
who was in India recently to "educate Indian business leaders
on the importance of compliance".
The anti-bribery provisions of FCPA prohibit "us persons"
from paying or offering to pay "anything of value to any
foreign official" with the "corrupt purpose" of
obtaining business. The penalties for violating the FCPA's anti-bribery
prohibitions are potentially severe. Both companies and individuals
indulging in corrupt practices will be subject to civil fines.
Other potential penalties include disqualification from us government
contracts and denial of export licences. "I have been interacting
with CEOs and CFOs of various Indian companies and have got the
impression that they all follow very stringent codes of ethics
and have checks and balances in place," she says, adding
that while many of them prohibit their own employees from bribing
government officials, they sometimes hire agents to do so. "This,
too, violates FCPA norms," she says.
Strict norms, but that's the price that companies have to pay
for enjoying the benefits of globalisation.
-Aman Malik
Pharma
Outsourcing to Grow Seven-fold
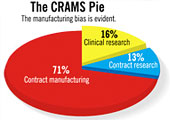 The
dynamics of the global pharma and life sciences industry continue
to favour outsourcing of research and manufacturing, and India
is one of the preferred low-cost destinations for this. India's
contract research and manufacturing services (crams) market was
valued at $895.44 million (Rs 4,029 crore) in 2006, a growth of
43 per cent over the previous year, says a study by Frost &
Sullivan, which expects the market to grow 33-34 per cent annually
on average to reach $6.6 billion (Rs 27,720 crore) by 2013. crams,
which has been contributing close to 8 per cent to the Indian
pharmaceutical industry's revenues, comprises contract research,
clinical research and contract manufacturing. The
dynamics of the global pharma and life sciences industry continue
to favour outsourcing of research and manufacturing, and India
is one of the preferred low-cost destinations for this. India's
contract research and manufacturing services (crams) market was
valued at $895.44 million (Rs 4,029 crore) in 2006, a growth of
43 per cent over the previous year, says a study by Frost &
Sullivan, which expects the market to grow 33-34 per cent annually
on average to reach $6.6 billion (Rs 27,720 crore) by 2013. crams,
which has been contributing close to 8 per cent to the Indian
pharmaceutical industry's revenues, comprises contract research,
clinical research and contract manufacturing.
"Indian contract manufacturers have traditionally been
strong in making active pharmaceutical ingredients (APIs) and
formulations. Now, we are seeing them developing competencies
in making oral solids and injectibles. This is a move up the value
chain," says Mahesh Sawant, Programme Manager (Healthcare
Practice), Frost & Sullivan. The study also points to India's
growing biology-based skills as opposed to chemistry-based capabilities.
In the clinical research space, which makes up 16 per cent of
the crams market (revenues: $143 million or Rs 600 crore), Indian
players have mostly been offering clinical trial facilities (mainly
bio-availability and bio-equivalence studies) to their foreign
clients, the market for which is set to grow "exponentially".
Despite greater business opportunities, not everyone is excited
about clinical trials and toxicology studies.
N. Raghuram, Professor of Biotechnology at Delhi's GGS Indraprastha
University, says: "Foreign drugmakers are outsourcing clinical
trials to India mainly because costs and compliance issues are
becoming prohibitive for them in the West. In India, however,
our capacity for complying with safeguards is very low, which
is a cause for concern." Sawant counters this saying that
most clinical trials outsourced to India are beyond the stage
where they can be a threat to the health of volunteers. In the
contract research arena (mostly pre-clinical R&D), whose revenues
were $116 million (Rs 522 crore) in 2006, the study sees chemistry-based
services continuing to drive business. Most common therapeutic
segments outsourced to India are oncology, infection control,
endocrinology and psychiatry.
-Kapil Bajaj
Realty
Stars
It's now the turn of real estate
companies to jump onto the celebrity endorsement bandwagon. Leading
realtors have roped in Amitabh Bachchan, Shah Rukh Khan, Rahul
Dravid, Ustad Amjad Ali Khan and several other stars to sell realty
dreams to India's consuming classes. The rationale: even a utilitarian
product like housing needs crutches. "It's unfair to single
out realty companies. Even a soap like Lux, which is as necessary
as a house, has been using celebrities to its advantage,"
argues Prabhakar Mundkur, COO, Percept H, the creative agency
behind DLF's Shah Rukh Khan commercial.
But Madhukar Kamath, Managing Director and CEO, Mudra Group,
dubs it a ridiculous idea. "It's probably to hide the lack
of product differentiation in real estate that celebrities are
being used." But what started as a niche has now become mainstream,
negating the very purpose for which these highly paid endorsers
are hired. So, it's back to square one.
-Tejeesh N.S. Behl
|






 The
dynamics of the global pharma and life sciences industry continue
to favour outsourcing of research and manufacturing, and India
is one of the preferred low-cost destinations for this. India's
contract research and manufacturing services (crams) market was
valued at $895.44 million (Rs 4,029 crore) in 2006, a growth of
43 per cent over the previous year, says a study by Frost &
Sullivan, which expects the market to grow 33-34 per cent annually
on average to reach $6.6 billion (Rs 27,720 crore) by 2013. crams,
which has been contributing close to 8 per cent to the Indian
pharmaceutical industry's revenues, comprises contract research,
clinical research and contract manufacturing.
The
dynamics of the global pharma and life sciences industry continue
to favour outsourcing of research and manufacturing, and India
is one of the preferred low-cost destinations for this. India's
contract research and manufacturing services (crams) market was
valued at $895.44 million (Rs 4,029 crore) in 2006, a growth of
43 per cent over the previous year, says a study by Frost &
Sullivan, which expects the market to grow 33-34 per cent annually
on average to reach $6.6 billion (Rs 27,720 crore) by 2013. crams,
which has been contributing close to 8 per cent to the Indian
pharmaceutical industry's revenues, comprises contract research,
clinical research and contract manufacturing. 
